Okay, before you go and soak your rechargeable batteries into tubs of egg yolk, you better read this article first. While batteries are of course a very crucial part of our enjoyment of our digital devices, the constant charging and discharging leads to deterioration over time. But scientists and researchers from Tsinghua University and MIT may have just discovered the solution to this problem: applying the “yolk and shell” principle may just save our dischargeable batteries.
Their study has been published in Nature Communications and may be the first step towards creating better lasting rechargeable batteries. The most widely-used ones are the lithium-ion batteries but they use graphite anodes which shrink and expand during the charging cycles, leading to an eventual deterioration. Aluminum, the alternative to graphite anodes, also experience this stress, plus the liquid electrolytes decompose when it touches aluminum.
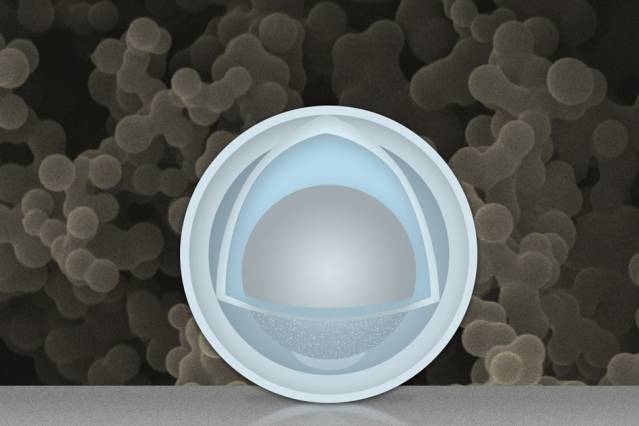
In their research, what they’ve discovered is that if you put a nanoparticle electrode with a “yolk” inside a solid shell and if you put a void between the two, then the “yolk” will have enough room to expand and contract. It will also not affect the stability and dimensions of the “shell”. They also say that the process they used to come up with this idea is “simple and scalable” so it shouldn’t pose a problem if any of the OEMs decide to duplicate the results onto an actual consumer product.
MIT Professor Ju Li, who did the study and wrote the paper together with 6 others, said that the materials that an be used is not expensive and the manufacturing method need not be so complicated and cost-heavy. Let’s see if something will eventually come out of this interesting approach to rechargeable batteries.
SOURCE: MIT







Someone make a video on how to do this
<-*&%^&%%^%^$% Get Ready For a home Business Now. You can Earn More in your spare time at your computer. I done this and now i have earned more and more every month Try This………. JobHome20. Please Complete The Site Address With CO m ->